Vitamin Mortality Meta-Analyses
[ Because nearly this entire page is based on meta-analyses, I'm ignoring my typical custom of coloring evidence from then. ]
This is a collection of meta-analyses examining the effect of vitamins on measures of mortality. I've ignored studies examining effects specifically on sick people, studies on specifically infants or pregnant women, or studies specifically on low-income countries.
todo Fortmann
Multivitamins
Multivitamin-multimineral supplementation and mortality: a meta-analysis of randomized controlled trials Macpherson
Randomized and controlled primary or secondary prevention trials were considered appropriate for review. To limit heterogeneity between the included trials, the following inclusion criteria were enforced: the trial must have been randomized and controlled, the participants must have been supplemented with a daily MVMM formulation in at least one study arm, the MVMM must have been taken orally and administered as a monotherapy within the treatment arm, the supplementation must have taken place for $1 y, and the cohort must not have been institutionalized or have had a terminal illness (tertiary prevention). Furthermore, each trial must have reported on the number of deaths in both the control and MVMM groups, or these data must have been made available on request. As recommended (14, 15), studies without deaths were excluded from the analyses
| Subgroup | Metric | Low | High |
| all | all-cause mortality | 0.94 | 1.02 |
| primary prevention trials | all-cause mortality | 0.89 | 1.00 |
| high-income countries | all-cause mortality | 0.95 | 1.04 |
| all | cancer mortality | 0.88 | 1.04 |
| all | vascular mortality | 0.93 | 1.09 |
Dietary supplements and risk of cause-specific death, cardiovascular disease, and cancer: a protocol for a systematic review and network meta-analysis of primary prevention trials. Schwingshackl
Studies were included if they met the following criteria: 1) randomized controlled design (identical placebo or no intervention) or trials of one supplement compared with another; 2) minimum intervention period of 12 mo; 3) primary prevention trial (defined as trials with the first occurrence of a chronic disease as the primary outcome); 4) mean age ≥18 y; 5) an intervention that used dietary supplements defined according to the Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002. The following dietary supplements were included according to previous systematic reviews and meta-analyses on dietary supplements and chronic diseases (9, 12, 16): vitamins [β-carotene; vitamins A, E, C (ascorbic acid), and D (cholecalciferol, ergocalciferol)]; B vitamins (thiamin, riboflavin, niacin, pyridoxine, cobalamin, folic acid); supplements containing a combination of different vitamins; FAs (n–3 FAs [EPA, DHA, α-linolenic acid (18:3n−3)]; n–6 FAs [linoleic acid (18:2n−6)]; monounsaturated fat (olive oil); minerals (magnesium, calcium, selenium, potassium, iron, zinc, copper, iodine); multiminerals; supplements containing combinations of both vitamins and minerals; protein (amino acids); fiber (psyllium, inulin, cellulose); probiotics; prebiotics; and synbiotics; 6) oral intake (modalities of supplement intake such as liquid, pill, capsule, tablet, drops, ampoule, powdered); and 7) assessment of clinical outcomes (“primary”: all-cause mortality; “secondary”: cardiovascular mortality, cancer mortality, cardiovascular incidence, and cancer incidence).
...
The exclusion criteria were as follows: 1) studies with a dietary or drug co-intervention that was not applied in all intervention or placebo and control groups; 2) studies with intravenous or parenteral administration of vitamins or minerals; 3) pregnant or lactating women; 4) mean age ≥70 y; 5) >75% of sample size assigned to secondary prevention trials [defined as trials undertaken to prevent recurrences or exacerbations of a disease that has already been diagnosed, such as in cancer survivors; survivors of myocardial infarction, stable or unstable angina pectoris, acute coronary insufficiency, coronary artery disease (verified by coronary angiography), stroke, hemodialysis, or chronic kidney disease; and subjects with the following diseases: gastrointestinal, neurological, ocular, dermatologic, rheumatoid, endocrinologic]; and 6) follow-up time not reported.
Note: for this study (for all vitamins), I only covered all-cause, cardiovascular, and cancer mortality.
Vitamin D
Dietary supplements and risk of cause-specific death, cardiovascular disease, and cancer: a protocol for a systematic review and network meta-analysis of primary prevention trials. Schwingshackl
| Subgroup | Metric | Low | High |
| any | all-cause mortality | 0.83 | 1.01 |
| Vitamin D alone | all-cause mortality | 0.04 | 3.20 |
| Vitamin D with others | all-cause mortality | 0.83 | 1.01 |
| dose < 500 IU/d | all-cause mortality | 0.83 | 1.01 |
| dose ≥ 500 IU/d | all-cause mortality | 0.39 | 1.45 |
| dose < 500 IU/d | cardiovascular mortality | 0.76 | 1.09 |
| dose < 500 IU/d, Vitamin D alone | cardiovascular mortality | 0.01 | 7.96 |
| dose < 500 IU/d, Vitamin D with others | cardiovascular mortality | 0.41 | 1.76 |
| dose < 500 IU/d | cancer mortality | 0.76 | 1.09 |
| dose < 500 IU/d, Vitamin D alone | cancer mortality | 0.01 | 7.96 |
| dose < 500 IU/d, Vitamin D with others | cancer mortality | 0.41 | 1.76 |
Vitamin D supplements and cancer incidence and mortality: a meta-analysis Keum
To be included, studies had to be a RCT providing information on the effect of vitamin D supplementation (with or without calcium supplementation) on total cancer incidence or mortality. When there were several publications from the same trial, the publication most fully covering the intervention period was selected.
| Subgroup | Metric | Low | High |
| all | cancer incidence | 0.94 | 1.06 |
| all | cancer mortality | 0.78 | 0.98 |
The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis Bolland
We excluded cluster randomised trials, trials of hydroxylated vitamin D or vitamin D analogues, trials that included other interventions only in the vitamin D group, trials of fortified dairy products, and trials in populations with chronic comorbidity other than osteoporosis or frailty (appendix).
| Subgroup | Metric | Low | High |
| vitamin D without calcium | heart attacks or ischaemic heart disease | 0.86 | 1.13 |
| vitamin D with calcium | heart attacks or ischaemic heart disease | 0.86 | 1.63 |
| all | heart attacks or ischaemic heart disease | 0.93 | 1.13 |
| vitamin D without calcium | stroke or cerebrovascular disease | 0.92 | 1.30 |
| vitamin D with calcium | stroke or cerebrovascular disease | 0.87 | 1.13 |
| all | stroke or cerebrovascular disease | 0.90 | 1.13 |
| vitamin D without calcium | cancer | 0.83 | 1.17 |
| vitamin D with calcium | cancer | 0.67 | 1.18 |
| all | cancer | 0.93 | 1.05 |
| all | heart attacks | 0.91 | 1.17 |
| all | ischaemic heart disease or cardiovascular disease | 0.78 | 1.62 |
| all | stroke | 0.88 | 1.13 |
| all | cerebrovascular disease | 0.82 | 1.34 |
| vitamin D without calcium | fractures | 0.88 | 1.08 |
| vitamin D with calcium | fractures | 0.85 | 0.99 |
| all | fractures | 0.88 | 1.02 |
| vitamin D without calcium | hip fractures | 0.97 | 1.27 |
| vitamin D with calcium | hip fractures | 0.74 | 0.96 |
| all | hip fractures | 0.86 | 1.08 |
| vitamin D without calcium | all-cause mortality | 0.92 | 1.01 |
| vitamin D with calcium | all-cause mortality | 0.89 | 1.02 |
| all | all-cause mortality | 0.93 | 1.00 |
Meta-analysis of long-term vitamin D supplementation on overall mortality Zheng
Types of studies. Randomized controlled trials evaluating an intervention with vitamin D were identified as part of the review, while review articles, commentaries, letters, observational studies were excluded.
Interventions. The intervention group was restricted to vitamin D alone or in combination with calcium treatment; the control group was placebo, no treatment or calcium only therapy. Studies of patients receiving active vitamin D and intramuscular injection of vitamin D were excluded from the review.
Outcome. The number of deaths was reported separately for the vitamin D treatment group and the control group. For articles with a large sample size, if the number of deaths was not reported by treatment, we tried to contact the authors to obtain the missing data.
| Subgroup | Metric | Low | High |
| < 3 year follow-up | all-cause mortality | 0.97 | 1.12 |
| ≥ 3 year follow-up | mortality | 0.90 | 0.98 |
| women | all-cause mortality | 0.83 | 1.00 |
| younger than 80 | all-cause mortality | 0.88 | 0.97 |
| older than 80 | all-cause mortality | 0.90 | 1.04 |
| dose ≤ 800 IU | all-cause mortality | 0.89 | 0.98 |
| dose > 800 IU | all-cause mortality | 0.89 | 1.03 |
| baseline of 25-hydroxyvitamin D less than 500 nmol/l | all-cause mortality | 0.89 | 0.98 |
| baseline of 25-hydroxyvitamin D greater than 500 nmol/l | all-cause mortality | 0.89 | 1.03 |
| treatment with cholecalcifrl | all-cause mortality | 0.89 | 0.97 |
| treatment with ergocalciferol | all-cause mortality | 0.90 | 1.06 |
| vitamin D with calcium | all-cause mortality | 0.88 | 0.99 |
| calcium | all-cause mortality | 0.91 | 1.03 |
| vitamin D alone | all-cause mortality | 0.86 | 1.00 |
| all | cancer mortality | 0.79 | 0.98 |
| all | cardiovascular mortality | 0.81 | 1.02 |
Extraskeletal effects of vitamin D in older adults: cardiovascular disease, mortality, mood, and cognition Barnard
Publications had to include patients ≥65 years of age who had ≥1 recorded measurement of 25(OH)D or were receiving vitamin D supplementation.
Note: This study examined a lot of hypotheses. I skipped many.
| Subgroup | Metric | Low | High |
| all | all-cause mortality | 0.83 | 1.01 |
| women less than 70 | all-cause mortality | 0.79 | 1.01 |
| women 70 or older | all-cause mortality | 0.80 | 1.12 |
| women 70 or older | CV | 0.78 | 1.32 |
| women 70 or older | coronary artery | 0.71 | 1.47 |
| women 70 or older | cerebrovascular | 0.72 | 2.01 |
| women 70 or older | cancer deaths | 0.65 | 1.12 |
Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials Gandini
For an article to be included in our analysis, it must have met the following criteria:
- To represent the principal published report on a randomized controlled trial evaluating an intervention with vitamin D. The addition of calcium supplements in the intervention group and the absence of a placebo for vitamin D in the control group (ie, an open-label trial) were not exclusion criteria.
- To be independent from other studies to avoid giving double weight to estimates derived from the same trial.
- To have deaths from any cause reported separately for the intervention and the control groups. If in an article the number of all-cause deaths was not reported by treatment group, we tried to contact corresponding authors to obtain the missing information.
- To have subjects randomized to the intervention and control groups on an individual basis. Cluster randomization (eg, a nursing home taken as a randomization unit) was not valid because mortality in a specific cluster could be increased by a health event (eg, an influenza epidemic) affecting this cluster and not the others.
- To have sufficient information to allow adequate estimation of the relative risks (RRs) and 95% confidence intervals (CIs) (ie, crude data or adjusted RRs and standard errors, 95% CIs, or P values) to estimate mortality risk after vitamin D intake vs placebo or control.
| Subgroup | Metric | Low | High |
| all | all-cause mortality | 0.87 | 0.99 |
| follow-up ≥ 3 years | all-cause mortality | 0.83 | 1.01 |
| follow-up < 3 years | all-cause mortality | 0.83 | 1.10 |
| dose ≥ 800 IU/d | all-cause mortality | 0.85 | 1.03 |
| dose between 300 and 799 IU/d | all-cause mortality | 0.82 | 1.03 |
| placebo-controlled trials | all-cause mortality | 0.86 | 0.98 |
| non-placebo-controlled trials | all-cause mortality | 0.84 | 1.45 |
| vitamin D and calcium | all-cause mortality | 0.86 | 1.01 |
| vitamin D only | all-cause mortality | 0.78 | 1.06 |
| D3 and not D2 | all-cause mortality | 0.87 | 0.98 |
Vitamin D status and ill health: a systematic review Boniol
| Subgroup | Metric | Low | High |
| all | Cardiovascular Diseases | 0.89 | 1.09 |
| all | Heart Attack | 0.93 | 1.13 |
| all | Stroke | 0.88 | 1.25 |
| all | All-cause Mortality | 0.93 | 0.96 |
Vitamin D and calcium: a systematic review of health outcomes Brendel Newberry
| Subgroup | Metric | Low | High | Study | Page |
| all | All-Cause Mortality | 0.92 | 1.02 | Brendel | 114 |
| all | All-Cause Mortality | 0.92 | 1.02 | Newberry | 114 |
They looked at cardiovascular events and cancer too (pg 48 and pg 61 of Brendel, pg 48 and pg 93 of Newberry), but only don't give any combined confidence intervals - only intervals for individual studies.
In the chart at the top of the vitamin D section, I only add one datapoint for Newberry since the analysis is very similar.
Antioxidants
Efficacy of antioxidant vitamins and selenium supplement in prostate cancer prevention: a meta-analysis of randomized controlled trials Efficacy of antioxidant vitamins and selenium supplement in prostate cancer prevention: a meta-analysis of randomized controlled trials
We included randomized and quasi-randomized trials (if a system involving dates of admission, odd or even date of birth, or hospital record numbers were used for the allocation of patients, these studies are known as quasi-randomized) that randomized participants to supplementation with antioxidants (β-carotene, vitamins A, C, E, α-tocopherol, and selenium, as different combinations or separately) versus placebo and reported the incidence or mortality of prostate cancer. We included participants from the general population, mainly with non-prostate diseases, and at high risk of developing prostate cancer.
We excluded studies in which prepared food containing antioxidant vitamins or selenium was given rather than a unique supplement of antioxidant vitamins or selenium. We also excluded studies in which the outcomes were not clearly defined. We excluded reviews letters, commentaries, and editorials if they did not contain original data
Efficacy of antioxidant supplementation in reducing primary cancer incidence and mortality: systematic review and meta-analysis Bardia
Eligible studies were trials with a minimum of 1 year of followup and that randomly assigned participants—who had no history of cancer (except skin cancer) nor precancerous lesions—to receive antioxidants or placebo. Eligible antioxidants were beta carotene, selenium, zinc, vitamin C (ascorbic acid), vitamin E (α-tocopherol), and lycopene alone or in combination with other antioxidant supplements, administered by mouth or parenterally. Ineligible trials used antioxidant supplements with undisclosed components or sought antioxidant supplementation through dietary increases in vegetables, fruits, or fiber. Also, trials reporting only site-specific cancer incidence or only cancer mortality, but not overall cancer incidence, were excluded.
| Subgroup | Metric | Low | High |
| all | cancer incidence | 0.94 | 1.04 |
| all | cancer mortality | 0.92 | 1.15 |
Reexamination of a meta-analysis of the effect of antioxidant supplementation on mortality and health in randomized trials Biesalski
This study starts with nearly the same sources as Mortality in randomized trials of antioxidant supplements for primary and secondary, but the authors conduct different analyses. They do not compute any new confidence intervals.
Effect of antioxidant vitamin supplementation on cardiovascular outcomes: a meta-analysis of randomized controlled trials Effect of antioxidant vitamin supplementation on cardiovascular outcomes: a meta-analysis of randomized controlled trials
We restricted our research based on randomized controlled trials, which contributed less confounding and bias than based on observational studies. All completed trials evaluating the effects of antioxidant vitamin on cardiovascular outcomes as compared to placebo, and providing at least 1 outcome as follows: major cardiovascular events, myocardial infarction, stroke, total death, or cardiac death.
| Subgroup | Metric | Low | High |
| all | cardiovascular events | 0.96 | 1.03 |
| all | heart attacks | 0.92 | 1.04 |
| all | stroke | 0.93 | 1.05 |
| all | all-cause mortality | 0.98 | 1.07 |
| all | cardiac events | 0.97 | 1.07 |
| all | revascularization risk | 0.95 | 1.05 |
| all | coronary heart disease | 0.87 | 1.05 |
| all | angina | 0.90 | 1.07 |
| all | congestive heart failure | 0.96 | 1.19 |
| all | ≥ 10000 patients | 0.91 | 1.05 |
| all | < 10000 patients | 0.88 | 1.14 |
| all | mean age ≥ 60 | 0.92 | 1.13 |
| all | mean age < 60 | 0.82 | 1.10 |
| all | men | 0.89 | 1.11 |
| all | women | 0.81 | 1.06 |
| all | men and women | 0.90 | 1.20 |
| all | healthy population | 0.89 | 1.05 |
| all | high risk for cardiovascular events | 0.92 | 1.10 |
| all | follow-up ≥ 6 months | 0.92 | 1.07 |
| all | follow-up < 6 months | 0.87 | 1.08 |
| all | Jadad score ≥ 4 | 0.92 | 1.06 |
| all | Jadad sore < 4 | 0.84 | 1.11 |
Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis
I can't find access to this.
Do antioxidants prevent colorectal cancer? A meta-analysis Pais
I can't find access to this.
Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: do we have evidence for lack of harm? Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: do we have evidence for lack of harm?
We considered for inclusion primary and secondary prevention randomized clinical trials in adults (aged ≥ 18 years) comparing beta-carotene, vitamin A, vitamin C, vitamin E, and selenium at any dose, duration, and route of administration versus placebo. The antioxidants could have been administered separately or in any combination, or in combination with other vitamins or trace elements without antioxidant function. Concomitant interventions were allowed when used equally in all the intervention groups of the trial.
For the present study we selected only the randomized primary or secondary prevention trials with low risk of bias where beta-carotene, vitamin A, and vitamin E were compared with placebo.
The analysis was done on a per-vitamin basis, so see those sections on this page for the metrics.
Beta carotene
Efficacy of antioxidant vitamins and selenium supplement in prostate cancer prevention: a meta-analysis of randomized controlled trials Efficacy of antioxidant vitamins and selenium supplement in prostate cancer prevention: a meta-analysis of randomized controlled trials
| Subgroup | Metric | Low | High |
| any | prostate cancer | 0.90 | 1.05 |
| beta-carotene and retinyl palmitate | prostate cancer | 0.90 | 1.16 |
| any | prostate cancer mortality | 0.87 | 1.65 |
| beta-carotene and retinyl palmitate | prostate cancer mortality | 0.59 | 1.19 |
Dietary supplements and risk of cause-specific death, cardiovascular disease, and cancer: a protocol for a systematic review and network meta-analysis of primary prevention trials. Schwingshackl
| Subgroup | Metric | Low | High |
| any | all-cause mortality | 0.96 | 1.09 |
| beta-carotene alone | all-cause mortality | 1.02 | 1.10 |
| beta-carotene with others | all-cause mortality | 0.92 | 1.11 |
| dose < 30 mg/d | all-cause mortality | 0.90 | 1.07 |
| dose ≥ 30 mg/d | all-cause mortality | 1.03 | 1.18 |
| any | cardiovascular mortality | 0.92 | 1.10 |
| beta-carotene alone | cardiovascular mortality | 0.93 | 1.16 |
| beta-carotene with others | cardiovascular mortality | 0.84 | 1.09 |
| dose < 30 mg/d | cardiovascular mortality | 0.85 | 1.10 |
| dose ≥ 30 mg/d | cardiovascular mortality | 0.93 | 1.21 |
| beta-carotene alone | cancer mortality | 0.89 | 1.13 |
| beta-carotene with others | cancer mortality | 0.81 | 1.47 |
| dose < 30 mg/d | cancer mortality | 0.84 | 1.14 |
| dose ≥ 30 mg/d | cancer mortality | 0.91 | 1.38 |
Efficacy of antioxidant supplementation in reducing primary cancer incidence and mortality: systematic review and meta-analysis Bardia
| Subgroup | Metric | Low | High |
| all | cancer incidence | 1.00 | 1.12 |
| smokers | cancer incidence | 1.03 | 1.18 |
| nonsmokers | cancer incidence | 0.92 | 1.10 |
| all | cancer mortality | 0.98 | 1.37 |
Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: do we have evidence for lack of harm? Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: do we have evidence for lack of harm?
| Subgroup | Metric | Low | High |
| Beta carotene, alone | all-cause mortality | 1.02 | 1.10 |
| any | all-cause mortality | 1.01 | 1.09 |
| dose ≤ 9.6 mg | all-cause mortality | 0.69 | 1.17 |
| dose > 9.6 mg | all-cause mortality | 1.02 | 1.09 |
A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease Mente
We only considered studies that followed up subjects for at least 1 year. Cohort studies had to include estimates of dietary intake using conventional dietary assessment tools (eg, food frequency questionnaires, food records, or 24-hour diet recall). Clinical trials had to be randomized and to compare dietary exposure with a control diet or a placebo. Crossover trials were excluded if plasma biomarkers or atherosclerotic indicators were not evaluated because coronary outcomes occurring after a crossover would be difficult to interpret.
| Subgroup | Metric | Low | High |
| Beta carotene supplements | all-cause mortality | 0.92 | 1.09 |
Vitamin E
Efficacy of antioxidant vitamins and selenium supplement in prostate cancer prevention: a meta-analysis of randomized controlled trials Efficacy of antioxidant vitamins and selenium supplement in prostate cancer prevention: a meta-analysis of randomized controlled trials
| Subgroup | Metric | Low | High |
| any | prostate cancer | 0.85 | 1.08 |
| vitamin E + selenium | prostate cancer | 0.92 | 1.19 |
| any | prostate cancer mortality | 0.58 | 1.24 |
Dietary supplements and risk of cause-specific death, cardiovascular disease, and cancer: a protocol for a systematic review and network meta-analysis of primary prevention trials. Schwingshackl
| Subgroup | Metric | Low | High |
| any | all-cause mortality | 0.99 | 1.05 |
| Vitamin E alone | all-cause mortality | 0.99 | 1.06 |
| Vitamin E with others | all-cause mortality | 0.32 | 1.06 |
| dose < 500 IU/d | all-cause mortality | 0.89 | 1.07 |
| dose ≥ 500 IU/d | all-cause mortality | 0.95 | 1.06 |
| any | cardiovascular mortality | 0.80 | 0.96 |
| Vitamin E alone | cardiovascular mortality | 0.79 | 0.98 |
| Vitamin E with others | cardiovascular mortality | 0.80 | 1.00 |
| dose < 500 IU/d | cardiovascular mortality | 0.76 | 0.99 |
| dose ≥ 500 IU/d | cardiovascular mortality | 0.77 | 1.01 |
| Vitamin E alone | cardiovascular mortality | 0.79 | 1.28 |
| Vitamin E with others | cardiovascular mortality | 0.79 | 1.08 |
| dose < 500 IU/d | cardiovascular mortality | 0.76 | 1.14 |
| dose ≥ 500 IU/d | cardiovascular mortality | 0.81 | 1.26 |
Vitamin E supplementation and mortality in healthy people: a meta-analysis of randomised controlled trials Curtis
To be included, studies had to meet pre-specified selection criteria as follow.
- Be a randomised, placebo-controlled trial with
- an intervention period of ≥6 months;
- no intervention co-supplements, i.e. other antioxidants or medications, concurrently with vitamin E unless balanced by a corresponding control arm that also included the additional antioxidant, supplement or medication
- Investigate the effect of any dose of synthetic (dl-alphatocopherol) or natural (d-alpha-tocopherol) oral vitamin E supplementation;
- Include adult men and/or non-pregnant women;
- Trial participants must be in good general health. On this basis, we included the following:
- Trials in which participants were recruited from the general population;
- Trials in which participants had non-systemic medical conditions, such as knee osteoarthritis;
- Trials in which otherwise healthy participants had risk factors for CVD, such as smoking, hypercholesterolaemia or atherosclerosis;
- Trials in which participants had chronic conditions, such as diabetes mellitus without serious comorbidities or complications.
- Be undertaken in highly or very highly developed countries, defined according to the United Nations Human Development Index;
- The number of deaths was available for intervention and control groups, and mortality was either a pre-specified primary or secondary outcome, or the methods indicated complete follow-up of participants or full ascertainment of deaths.
The following types of trials were excluded:
- Trials that selected patients on the basis of having a serious disease that may result in lower life expectancy, such as cancer, recent myocardial infarction, chronic infectious disease, Alzheimer’s disease or end stage renal disease;
- Trials undertaken in developing countries, due to the higher likelihood of participants having underlying nutritional deficiencies;
- Trials where no deaths were reported.
| Subgroup | Metric | Low | High |
| all | all-cause mortality | 0.97 | 1.05 |
| synthetic | all-cause mortality | 0.96 | 1.05 |
| natural | all-cause mortality | 0.96 | 1.08 |
| < 400 | all-cause mortality | 0.97 | 1.07 |
| ≥ 400 | all-cause mortality | 0.94 | 1.05 |
| < 3 years | all-cause mortality | 0.46 | 1.58 |
| ≥ 3 years | all-cause mortality | 0.97 | 1.05 |
| mortality was pre-specified | all-cause mortality | 0.97 | 1.05 |
| mortality was not pre-specified | all-cause mortality | 0.40 | 1.26 |
| parallel group study | all-cause mortality | 0.68 | 1.58 |
| factorial study | all-cause mortality | 0.97 | 1.05 |
| high study quality | all-cause mortality | 0.96 | 1.05 |
| medium/low study quality | all-cause mortality | 0.96 | 1.08 |
A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease Mente
| Subgroup | Metric | Low | High |
| Vitamin E supplements | all-cause mortality | 0.84 | 1.01 |
Meta-analysis: low-dose intake of vitamin E combined with other vitamins or minerals may decrease all-cause mortality Meta-analysis: low-dose intake of vitamin E combined with other vitamins or minerals may decrease all-cause mortality
The following inclusion criteria were used to select studies for this analysis (1) RCTs evaluating the efficacy of vitamin E; (2) assessment of vitamin E intake alone or combined with at least one of the following vitamins or minerals, which have been largely reported to interact with vitamin E: vitamin C, β-carotene, selenium and zinc; (3) a study sample limited to men or non-pregnant women; (4) minimal vitamin E supplementation and a follow-up period of 1 y; and (5) a minimum of 10 deaths in trial...Trials with a Jadad score of 4 or more were included.
| Subgroup | Metric | Low | High |
| all | all-cause mortality | 0.89 | 1.06 |
| dose < 400 IU/d | all-cause mortality | 0.86 | 0.98 |
| dose ≥ 400 IU/d | all-cause mortality | 0.81 | 2.03 |
| age < 65 y old | all-cause mortality | 0.84 | 1.04 |
| age ≥ 65 y oldall | all-cause mortality | 0.94 | 1.19 |
| people without probably or confirmed diseases | all-cause mortality | 0.86 | 0.99 |
| unhealthy individuals with probably or confirmed diseases | mortality | 0.86 | 1.21 |
| short follow-up | all-cause mortality | 0.87 | 1.15 |
| long follow-up | all-cause mortality | 0.94 | 1.03 |
| vitamin E alone | all-cause mortality | 0.97 | 1.04 |
| vitamin E alone; dose < 400 IU/d | all-cause mortality | 0.96 | 1.05 |
| vitamin E alone; dose ≥ 400 IU/d | all-cause mortality | 0.95 | 1.06 |
| vitamin E alone; age < 65 | all-cause mortality | 0.97 | 1.04 |
| vitamin E alone; age ≥ 65 | all-cause mortality | 0.92 | 1.09 |
| vitamin E alone; people without probably or confirmed diseases | all-cause mortality | 0.97 | 1.05 |
| vitamin E alone; unhealthy individuals with probably or confirmed diseases | all-cause mortality | 0.93 | 1.06 |
| vitamin E alone; short follow-up | all-cause mortality | 0.88 | 1.06 |
| vitamin E alone; long follow-up | all-cause mortality | 0.98 | 1.05 |
Bayesian model averaging in meta-analysis: vitamin E supplementation and mortality Berry
TODO
Efficacy of antioxidant supplementation in reducing primary cancer incidence and mortality: systematic review and meta-analysis Bardia
| Subgroup | Metric | Low | High |
| all | cancer incidence | 0.94 | 1.04 |
| men | cancer mortality | 0.97 | 1.12 |
Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: do we have evidence for lack of harm? Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: do we have evidence for lack of harm?
| Subgroup | Metric | Low | High |
| vitamin E, alone | all-cause mortality | 0.98 | 1.05 |
| any | all-cause mortality | 1.00 | 1.05 |
| dose ≤ 15 mg | all-cause mortality | 0.51 | 3.46 |
| dose > 15 mg | all-cause mortality | 1.00 | 1.05 |
Vitamin E and all-cause mortality: a meta-analysis Abner
Inclusion criteria were (1) peer review (indicated by publication in a peer-reviewed journal), (2) randomized treatment conditions, (3) comparator arms, (4) adult participants (excluding pregnant women), (5) parallel or factorial designs, and (6) the assignment of participants to supplemental vitamin E, taken orally, alone or in combination with other drugs and supplements for at least one year (Figure 1).
| Subgroup | Metric | Low | High |
| any | all-cause mortality | 0.98 | 1.02 |
| double-blind; placebo-controlled | all-cause mortality | 0.99 | 1.03 |
The role of vitamin E in the prevention of cancer: a meta-analysis of randomized controlled trials The role of vitamin E in the prevention of cancer: a meta-analysis of randomized controlled trials.
Data sources were randomized controlled trials (RCTs) in which outcomes related to cancer prevention that were associated with the intake of vitamin E supplements alone or with other supplements were compared to a control group (placebo or control). Participants in studies were adults of either sex (18 years or older). Types of interventions were vitamin E alone or with other supplements versus placebo or no intervention. Supplementation was in capsule or tablet form, to be consumed by mouth.
| Subgroup | Metric | Low | High |
| any | all-cause mortality | 0.96 | 1.03 |
| any | cancer mortality | 0.95 | 1.09 |
| any | cancer incidence | 0.94 | 1.02 |
| any | stomach cancer | 0.90 | 1.15 |
| any | lung cancer | 0.88 | 1.19 |
| any | colorectal cancer | 0.81 | 1.12 |
| any | prostate cancer | 0.74 | 0.96 |
| any | breast cancer | 0.90 | 1.10 |
| any | esophageal cancer | 0.88 | 1.14 |
| any | hematological cancer | 0.71 | 1.33 |
| any | urinary tract cancer | 0.84 | 1.84 |
| vitamin E alone | all-cause mortality | 0.94 | 1.05 |
| vitamin E alone | colorectal cancer | 0.79 | 1.39 |
| vitamin E alone | prostate cancer | 0.70 | 1.06 |
| vitamin E alone | total cancer | 0.90 | 1.12 |
| vitamin E alone | cancer mortality | 0.83 | 1.26 |
| vitamin E with other supplements | all-cause mortality | 0.96 | 1.03 |
| vitamin E with other supplements | total cancer | 0.92 | 1.01 |
| vitamin E with other supplements | cancer mortality | 0.93 | 1.08 |
| vitamin E with other supplements | prostate cancer | 0.67 | 0.93 |
| dose ≥ 300 mg/d | all-cause mortality | 0.97 | 1.06 |
| dose ≥ 300 mg/d | cancer mortality | 0.92 | 1.17 |
| dose ≥ 300 mg/d | cancer incidence | 0.92 | 1.05 |
| dose ≥ 300 mg/d | lung cancer | 0.81 | 1.16 |
| dose ≥ 300 mg/d | prostate cancer | 0.79 | 1.11 |
| dose < 300 mg/d | all-cause mortality | 0.92 | 1.02 |
| dose < 300 mg/d | cancer mortality | 0.90 | 1.06 |
| dose < 300 mg/d | cancer incidence | 0.91 | 1.01 |
| dose < 300 mg/d | stomach cancer | 0.84 | 1.10 |
| dose < 300 mg/d | lung cancer | 0.85 | 1.10 |
| dose < 300 mg/d | prostate cancer | 0.55 | 0.87 |
Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality Miller
Our prespecified inclusion criteria were 1) random allocation of participants, 2) use of vitamin E supplementation alone or combined with other vitamins or minerals, 3) presence of a control or placebo group, 4) study sample limited to men or nonpregnant women, 5) duration of vitamin E supplementation and follow-up longer than 1 year, and 6) occurrence of at least 10 deaths in the trial.
| Subgroup | Metric | Low | High |
| any | all-cause mortality | 0.98 | 1.04 |
| dose < 400 IU/d | all-cause mortality | 0.96 | 1.01 |
| dose ≥ 400 IU/d | all-cause mortality | 1.01 | 1.07 |
| "adjusted for other vitamins or minerals" | all-cause mortality | 0. | 1. |
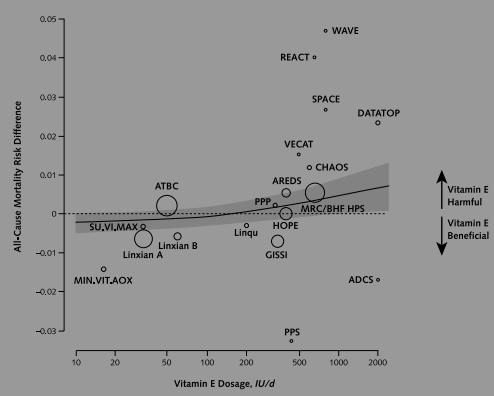
Effect of supplemental vitamin E for the prevention and treatment of cardiovascular disease Morton
| Subgroup | Metric | Low | High |
| vitamin E alone | all-cause mortality | 0.84 | 1.10 |
| vitamin E alone | cardiovascular mortality | 0.80 | 1.19 |
| vitamin E in combination | cardiovascular mortality | 0.81 | 1.32 |
| vitamin E alone | fatal heart attacks | 0.74 | 1.27 |
| vitamin E in combination | fatal heart attacks | 0.77 | 1.37 |
| vitamin E alone | nonfatal heart attacks | 0.51 | 1.02 |
| vitamin E in combination | nonfatal heart attacks | 0.89 | 1.1.0 |
The role of vitamin E in the prevention of coronary events and stroke. Meta-analysis of randomized controlled trials The role of vitamin E in the prevention of coronary events and stroke. Meta-analysis of randomized controlled trials
I couldn't find the text of this study.
Vitamin A
Dietary supplements and risk of cause-specific death, cardiovascular disease, and cancer: a protocol for a systematic review and network meta-analysis of primary prevention trials. Schwingshackl
| Subgroup | Metric | Low | High |
| any | all-cause mortality | 0.97 | 1.16 |
| Vitamin A alone | all-cause mortality | 0.81 | 1.65 |
| Vitamin A with others | all-cause mortality | 0.91 | 1.20 |
| dose < 25,000 IU/d | all-cause mortality | 0.84 | 1.08 |
| dose ≥ 25,000 IU/d | all-cause mortality | 1.04 | 1.21 |
| any | cardiovascular mortality | 0.78 | 1.21 |
| Vitamin A alone | cardiovascular mortality | 0.66 | 1.09 |
| Vitamin A with others | cardiovascular mortality | 0.92 | 1.24 |
| any | cardiovascular mortality | 0.82 | 1.43 |
| Vitamin A alone | cardiovascular mortality | 0.74 | 1.13 |
| Vitamin A with others | cardiovascular mortality | 1.05 | 1.47 |
Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age
TODO: Fill in ?sRandomised controlled trials (RCTs) and cluster-RCTs evaluating the effect of synthetic VAS in children aged six months to five years living in the community. We excluded studies involving children in hospital and children with disease or infection. We also excluded studies evaluating the effects of food fortification, consumption of vitamin A rich foods, or beta-carotene supplementation.
| Subgroup | Metric | Low | High |
| any | all-cause mortality | 0.83 | 0.93 |
| ? | all-cause mortality | 0.75 | 0.92 |
| ? | all-cause mortality | 0.81 | 0.97 |
| Asia | all-cause mortality | 0.84 | 0.96 |
| Africa | all-cause mortality | 0.75 | 0.98 |
| Latin America | all-cause mortality | 0.14 | 7.08 |
| 6 - 12 months old | all-cause mortality | 0.43 | 0.82 |
| 1 year to 5 years old | all-cause mortality | 0.57 | 0.81 |
| boys | all-cause mortality | 0.89 | 1.04 |
| girls | all-cause mortality | 0.84 | 0.97 |
| countries with high child mortality | all-cause mortality | 0.84 | 0.94 |
| countries with low child mortality | all-cause mortality | 0.14 | 7.08 |
| any | diarrhea mortality | 0.79 | 0.98 |
| ? | diarrhea mortality | 0.61 | 0.95 |
| any | measles mortality | 0.69 | 1.11 |
| ? | measles mortality | 0.52 | 1.37 |
| any | meningitis mortality | 0.17 | 1.88 |
| ? | meningitis mortality | 0.22 | 153.23 |
| any | lower respiratory tract infection mortality | 0.86 | 1.12 |
| ? | lower respiratory tract infection mortality | 0.40 | 1.10 |
| any | measles | 0.82 | 0.87 |
| ? | measles | 0.89 | 0.96 |
| any | measles | 0.37 | 0.67 |
| any | malaria incidence | 0.60 | 0.88 |
| ? | malaria prevalence | 0.41 | 1.28 |
| any | lower respiratory tract infection | 0.92 | 1.06 |
| ? | lower respiratory tract infection | 0.89 | 1.04 |
| ? | lower respiratory tract infection | 0.45 | 0.81 |
| any | Bitot's spots | 0.33 | 0.53 |
| ? | Bitot's spots | 0.33 | 0.56 |
| any | night blindness | 0.17 | 0.52 |
| any | xerophthalmia | 0.70 | 1.03 |
| ? | xerophthalmia | 0.72 | 1.07 |
| any | vomiting | 1.44 | 2.69 |
| any | bulging fontanelle | 0.74 | 2.08 |
| any | vitamin A deficiency | 0.65 | 0.78 |
| any | vitamin A serum levels | 0.22 | 0.30 |
| any | vitamin A serum levels | 0.37 | 0.53 |
Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: do we have evidence for lack of harm? Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: do we have evidence for lack of harm?
| Subgroup | Metric | Low | High |
| vitamin A, alone | all-cause mortality | 0.83 | 1.68 |
| any | all-cause mortality | 0.97 | 1.18 |
| dose ≤ 800 µg | all-cause mortality | 0.65 | 1.69 |
| dose > 800 µg | all-cause mortality | 0.97 | 1.19 |
Vitamin A supplementation in infectious diseases: a meta-analysis Glasziou
A report was dropped from further analysis if the study did not include concurrent controls or contained no original data or if the report did not address mortality, respiratory disease, or diarrhoea. Several trials that looked at cancer prevention were not considered within the scope of this analysis. On the basis of these initial broad criteria, clearly irrelevant articles were discarded after consideration by a single reviewer.
...
Papers that did not include a control group, were not randomised, did not allow calculation of "intention to treat" results, or did not collect information on mortality or the incidence of respiratory or gastrointestinal infection were excluded.
| Subgroup | Metric | Low | High |
| any | all-cause mortality | 0.62 | 0.79 |
| children in hospitals with measles | all-cause mortality | 0.15 | 0.77 |
| children in hospitals with measles | respiratory mortality | 0.10 | 0.85 |
| community studies | all-cause mortality | 0.64 | 0.86 |
| community trials | diarrhoeal mortality | 0.50 | 0.76 |
| community trials | measles mortality | 0.23 | 0.87 |
| community trials | mortality besides diarrhea, respiratory causes, orr measles | 0.51 | 0.85 |
Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis
TODO: Record cause-specific mortality and morbidities.Types of trials—Randomised controlled trials including cluster trials and factorial trials were included irrespective of publication status or language.
Types of participants—At the time of recruitment, children had to be aged 6 months to 5 years and apparently healthy. Children in hospital at the time of recruitment were excluded.
Types of interventions—Included studies examined synthetic oral vitamin A supplementation compared with no treatment or placebo, irrespective of dose or frequency. Studies of food fortification and β carotene supplementation were excluded as their effects can differ.
| Subgroup | Metric | Low | High |
| completed trials | all-cause mortality | 0.69 | 0.83 |
| completed trials lasting more than 13 months | all-cause mortality | 0.64 | 0.88 |
| complete and incomplete trials | all-cause mortality | 0.84 | 0.94 |
| Asia | all-cause mortality | 0.61 | 0.79 |
| Africa | all-cause mortality | 0.73 | 0.98 |
| Latin America | all-cause mortality | 0.14 | 7.08 |
| 6-12 months old | all-cause mortality | 0.43 | 0.82 |
| 1-5 years old | all-cause mortality | 0.57 | 0.82 |
| boys | all-cause mortality | 0.66 | 0.97 |
| girls | all-cause mortality | 0.65 | 0.95 |
| small frequent doses | all-cause mortality | 0.30 | 0.71 |
| doses every 4-6 months | all-cause mortality | 0.72 | 0.90 |
| one-time dose | all-cause mortality | 0.52 | 0.83 |
Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age
TODOB Vitamins
Meta-analysis of B vitamin supplementation on plasma homocysteine, cardiovascular and all-cause mortality Huang
Studies were eligible for inclusion if (1) the study design was a randomized controlled trial; (2) the relative risk (RR) or the number of events for CVD, coronary heart disease (CHD), stroke, and/or all-cause mortality that occurred during the study was reported by intervention and control groups; (3) the intervention was B vitamins; and (4) there was no limitation on the intervention duration.
| Subgroup | Metric | Low | High |
| any | CVD | 0.94 | 1.03 |
| any | CHD | 0.92 | 1.05 |
| any | heart attacks | 0.90 | 1.05 |
| any | strokes | 0.82 | 0.95 |
| any | cardiovascular mortality | 0.91 | 1.02 |
| any | all-cause mortality | 0.95 | 1.04 |
A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease Mente
| Subgroup | Metric | Low | High |
| folate supplements | all-cause mortality | 0.91 | 1.06 |
Vitamin C
Efficacy of antioxidant vitamins and selenium supplement in prostate cancer prevention: a meta-analysis of randomized controlled trials Efficacy of antioxidant vitamins and selenium supplement in prostate cancer prevention: a meta-analysis of randomized controlled trials
| Subgroup | Metric | Low | High |
| any | prostate cancer | 0.91 | 1.06 |
| any | prostate cancer mortality | 0.92 | 2.29 |
Dietary supplements and risk of cause-specific death, cardiovascular disease, and cancer: a protocol for a systematic review and network meta-analysis of primary prevention trials. Schwingshackl
| Subgroup | Metric | Low | High |
| any | all-cause mortality | 0.91 | 1.05 |
| Vitamin C alone | all-cause mortality | 0.91 | 1.11 |
| Vitamin C with others | all-cause mortality | 0.91 | 1.05 |
| dose < 500 IU/d | all-cause mortality | 0.83 | 1.04 |
| dose ≥ 500 IU/d | all-cause mortality | 0.93 | 1.09 |
| any | cardiovascular mortality | 0.82 | 1.13 |
| Vitamin C alone | cardiovascular mortality | 0.83 | 1.35 |
| Vitamin C with others | cardiovascular mortality | 0.80 | 1.12 |
| dose < 500 IU/d | cardiovascular mortality | 0.68 | 1.12 |
| dose ≥ 500 IU/d | cardiovascular mortality | 0.84 | 1.26 |
| Vitamin C alone | cardiovascular mortality | 0.81 | 1.21 |
| dose < 500 IU/d | cardiovascular mortality | 0.78 | 1.18 |
| dose ≥ 500 IU/d | cardiovascular mortality | 0.73 | 2.54 |
Adjuvant administration of vitamin C improves mortality of patients with sepsis and septic shock: a systems review and meta-analysis Adjuvant administration of vitamin C improves mortality of patients with sepsis and septic shock: a systems review and meta-analysis
We included trials with the following features:
- Type of trials: randomized controlled clinical trials and retrospective studies.
- Population: trials included adult population with sepsis or septic shock.
- Intervention: patients submitted to vitamin C for sepsis therapy.
- Comparison: placebo for sepsis therapy.
- Outcome: the primary outcome was 28-day mortality.
Trials with the following features were excluded:
- They were not published in English.
- They were not published as original articles.
- They did not use adult patients.
- They did not administrate vitamin C for sepsis therapy.
- They included no data on mortality in patients with sepsis or septic shock.
- Full-text articles were not available.
| Subgroup | Metric | Low | High |
| all | all-cause mortality | 0.16 | 1.73 |
| dose > 50 mg/kg/d, severe sepsis patients | all-cause mortality | 0.16 | 0.94 |
| dose < 50 mg/kg/d, severe sepsis patients | all-cause mortality | 0.28 | 3.58 |
| severe sepsis patients | all-cause mortality | 0.26 | 1.09 |
| septic shock patients | ICU duration | 4.91 | 1.98 |
A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease Mente
| Subgroup | Metric | Low | High |
| Vitamin C supplements | all-cause mortality | 0.70 | 1.25 |
Efficacy of vitamin C supplements in prevention of cancer: a meta-analysis of randomized controlled trials Efficacy of vitamin C supplements in prevention of cancer: a meta-analysis of randomized controlled trials
We included only RCTs that both reported the efficacy of vitamin C supplementation on cancer prevention and compared an intervention group with a control group. The main outcome measures included cancer incidence and mortality.
| Subgroup | Metric | Low | High |
| any | cancer incidence | 0.95 | 1.05 |
| < 500 mg | all-cause mortality | 0.91 | 1.05 |
| ≥ 500 mg | all-cause mortality | 0.95 | 1.10 |
| Vitamin C only | all-cause mortality | 0.95 | 1.10 |
| Combination with other supplements | all-cause mortality | 0.91 | 1.04 |
| follow-up < 7 years | all-cause mortality | 0.92 | 1.07 |
| follow-up ≥ 7 years | all-cause mortality | 0.94 | 1.07 |
| high methodological quality | all-cause mortality | 0.91 | 1.05 |
| low methodological quality | all-cause mortality | 0.95 | 1.10 |
| any | cancer incidence | 0.95 | 1.05 |
| any | cancer mortality | 0.96 | 1.17 |
| funded by non-pharmaceutical | all-cause mortality | 0.60 | 2.40 |
| funded by pharmaceutical | all-cause mortality | 0.96 | 1.07 |
| male | all-cause mortality | 0.62 | 1.20 |
| female | all-cause mortality | 0.96 | 1.23 |
| never smoked | all-cause mortality | 0.95 | 1.18 |
| former smoker | all-cause mortality | 0.90 | 1.11 |
| current smoker | all-cause mortality | 0.64 | 1.47 |
| China | all-cause mortality | 0.90 | 1.24 |
| USA | all-cause mortality | 0.95 | 1.10 |
| France | all-cause mortality | 0.77 | 1.07 |
| UK | all-cause mortality | 0.89 | 1.08 |
| any | respiratory cancer | 0.73 | 1.53 |
| any | haematological cancer | 0.87 | 1.28 |
| any | skin mortality | 0.81 | 1.11 |
| any | stomach mortality | 0.93 | 1.51 |
| any | esophageal mortality | 0.78 | 1.24 |
| any | cccolorectal mortality | 0.64 | 1.10 |
| any | breast mortality | 0.87 | 1.25 |
| any | pancreas mortality | 0.58 | 3.20 |
| any | genitourinary mortality | 0.76 | 1.02 |
| any | prostate mortality | 0.90 | 1.14 |
| any | bladder mortality | 0.53 | 1.34 |
| any | uterine mortality | 0.49 | 1.48 |
| any | ovary mortality | 0.36 | 1.93 |
| any | thyroid mortality | 0.63 | 2.97 |
| any | oral mortality | 0.13 | 2.00 |
| any | central nervous system mortality | 0.55 | 3.42 |
Folic Acid
Dietary supplements and risk of cause-specific death, cardiovascular disease, and cancer: a protocol for a systematic review and network meta-analysis of primary prevention trials. Schwingshackl
| Subgroup | Metric | Low | High |
| any | all-cause mortality | 0.75 | 1.23 |
| Folic acid alone | all-cause mortality | 0.60 | 1.99 |
| Folic acid with others | all-cause mortality | 0.03 | 2.22 |
| dose < 500 IU/d | all-cause mortality | 0.38 | 5.79 |
| dose ≥ 500 IU/d | all-cause mortality | 0.59 | 1.41 |
| Folic acid alone, dose ≥ 5 mg/d | cardiovascular mortality | 0.66 | 1.53 |
Efficacy of folic acid supplementation in stroke prevention: a meta-analysis Efficacy of folic acid supplementation in stroke prevention: a meta-analysis
TODOVitamin K
All the meta-analyses I found relating vitamin K to mortality were conducted on ill people being treated with vitamin K antagonists.